De Broglie Wavelength
Question 3.
Use Maple, to calculate the de Broglie wavelengths of the following.
(a) A nitrogen molecule at room temperature. Use the equipartition principle to help.
The equipartition principle states that at thermal equilibrium the amount of energy is equal to ½*k*T for a gas, where k is the Boltzmann constant.
We set our constants in Maple using room temperature at 23°C and m is the mass of nitrogen:
Planck Constant
>
![]()
![]()
Boltzmann Constant
>
![]()
![]()
Room Temp.
>
![]()
![]()
Mass of Nitrogen
>
![]()
![]()
Then we find KE using the equipartition principle that was explained above:
>
![]()
![]()
Finally solve for the wavelength using the equation from question 2:
>
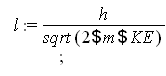
![]()
This shows the wavelength in meters of a nitrogen molecule at room temperature.
(b) A 1.5 g bullet traveling at 650 mi/hr.
Here we convert mi/hr to m/s:
>
![]()

And set this to the variable v:
>
![]()
![]()
Change g to kg:
>
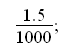
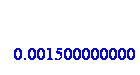
And set to variable m:
>
![]()
![]()
Using the equation for kinetic energy we figure out KE:
>
![]()
![]()
Finally determine the wavelength using the equation from question 2:
>
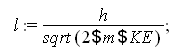
![]()
This answer shows us the wavelength of a 1.5 g bullet when it is traveling at 650 mi/hr. The wavelength of the bullet is even smaller than that of the nitrogen molecule at room temperature which means although the bullet has a wavelength there is no way we could see it with the human eye.