|
|
Plant Species Biology
Volume 15 Issue 3 Page 281 -
December 2000 |
| |
| Reproductive biology of a North
American subalpine plant: Corydalis caseana A. Gray ssp.
brandegei (S. Watson) G. B. Ownbey |
| Joan E. Maloof |
|
Abstract
Corydalis caseana ssp. brandegei (Fumariaceae) is a
perennial plant that grows in moist, subalpine regions of south
central Colorado, USA. Prior to this study, nothing was known of its
reproductive biology. The most numerous visitors (59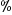 ), and
the only known pollinators, were long-tongued bumblebees (Bombus
appositus). Twenty-nine percent of visits were from
short-tongued nectar-robbing bumblebees (Bombus
occidentalis). Hummingbirds also visited the flowers but they
did not pollinate them. Corydalis caseana flowers remained
open and in good condition for approximately 4 days. During that
time, in the absence of visitors, nectar containing 35 ), and
the only known pollinators, were long-tongued bumblebees (Bombus
appositus). Twenty-nine percent of visits were from
short-tongued nectar-robbing bumblebees (Bombus
occidentalis). Hummingbirds also visited the flowers but they
did not pollinate them. Corydalis caseana flowers remained
open and in good condition for approximately 4 days. During that
time, in the absence of visitors, nectar containing 35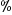 sugar
accumulated at a rate of approximately 1 sugar
accumulated at a rate of approximately 1  L per day.
Corydalis caseana has a mixed-mating system. It is
self-fertile, but the self-fertilized flowers produce fewer seeds
per fruit than the outcrossed flowers (a mean of 2.9 compared with a
mean of 4.7). Results suggest a possibility of inbreeding
depression. L per day.
Corydalis caseana has a mixed-mating system. It is
self-fertile, but the self-fertilized flowers produce fewer seeds
per fruit than the outcrossed flowers (a mean of 2.9 compared with a
mean of 4.7). Results suggest a possibility of inbreeding
depression. |
|
|
|
Corydalis caseana A. Gray (Fumariaceae) is an herbaceous,
perennial plant that usually grows in or near a source of fresh
water such as a small creek or snowmelt drainage. The mature plant
ranges from less than 0.5 m to over 2 m tall, but it is most
commonly approximately 1 m tall. It has glaucous, dissected, leaves
and multiple inflorescences of pink to white flowers. There are five
subspecies of C. caseana scattered across the mountainous
areas of the western United States. All of the subspecies are
geographically separated from each other, and differ morphologically
in minor, but perceptible ways, such as branching of the
inflorescence, height at maturity, typical flower color and the size
and shape of the outer petals. These details are described by Stern
(1998) and Ownbey
(1947).
Prior to this study, nothing was known about either the breeding
system or the pollinators of Corydalis caseana (A. Gray) ssp.
brandegei (S. Watson). In this study I determined the
phenology of the flowers, the breeding system, the identity of the
major flower visitors, pollinators and nectar robbers, the rate and
amount of nectar produced and the concentration of the nectar.
|
|
|
|
The plant
The subspecies I studied, C. caseana brandegei, grows in
central Colorado, USA, most commonly around altitudes of 3000 m. The
plant is rate, but where it does occur, it is locally abundant,
sometimes forming large, almost monospecific, patches containing
thousands of plants. With the onset of winter, all above-ground
parts die back. In spring, as the snow melts, new shoots emerge.
Seedlings germinate in the spring and develop a taproot that grows
larger every year. Each year a taller, single stem with more
leaflets is produced until the plant is mature enough to begin
flowering. Eventually the large taproot will give rise to more
stems, and these too must mature before they begin flowering. A
similar pattern has been described in a closely related species,
Corydalis aquae-gelidae ( Goldenberg
1992), which begins flowering about 7 years after germination (
Goldenberg
& Zobel 1997). An old plant may have up to 20 stems emerging
in close proximity to each other. A typical mature stem will have a
terminal racemose inflorescence that has up to 70 flowers and
numerous secondary racemes that may have from 5 to 40 flowers each.
Figure
1 shows the structure of the flowers. The corolla has four
petals. The inner petals are fused at the tip and they conceal the
anthers and stigma. The anthers are appressed to the subplanate
stigma. The outer petals are rotated 90° from the inner petals and
form the lips. The upper petal extends posteriorly to form a nectar
spur. When large visitors, such as bumblebees, collect nectar from
the front of the flower, the fused inner petals are depressed and
the reproductive organs are exposed through an open slit ( Fig.
1). The inner petals have a hinge-like structure to facilitate
this action. After the visitor leaves, the inner petals resume their
original position.
Fruits dehisce explosively as soon as the seeds mature,
approximately 20 days after pollination. |
|
Study site
This research was conducted in the vicinity of the Rocky Mountain
Biological Laboratory in Gothic, Colorado (38°50 N, 106°50 N, 106°50 W). Most
observations were carried out in Washington Gulch (2940 m, 38°56 W). Most
observations were carried out in Washington Gulch (2940 m, 38°56 N,
107°01 N,
107°01 W) in a subalpine meadow approximately
2 km from the laboratory. Additional pollinator observations were
conducted at Kebler Pass (38°50 W) in a subalpine meadow approximately
2 km from the laboratory. Additional pollinator observations were
conducted at Kebler Pass (38°50 N, 107°06 N, 107°06 W), 16 km from the
laboratory, in a series of eight meadows beginning at the top of the
pass (altitude 3050 m) and continuing 880 m in a north-west
direction to an altitude of 2975 m. W), 16 km from the
laboratory, in a series of eight meadows beginning at the top of the
pass (altitude 3050 m) and continuing 880 m in a north-west
direction to an altitude of 2975 m. |
|
Determination of flower phenology
In 1996, a representative sample of eight inflorescences on eight
different plants was chosen for this study. The plants were located
within 10 m of each other at the Washington Gulch site. Each
inflorescence had between 10 and 20 flower buds. Every bud or flower
(N = 101) was observed on 25, 26, 27, 28, 29 and 30 June and
on 1, 2, 3, 5, 7, 9, 11, 12, 15 and 17 July. The condition of each
flower was described according to the following categories: (i)
closed (bud stage); (ii) open, without holes from nectar robbers;
(iii) open, with holes from nectar robbers; (iv) flower with brown
spots or wilted; (v) flower fallen, no fruit forming; and (vi) fruit
forming. All of the observed flowers were in bud stage when the
study began, and all were either in fruit or had fallen from the
plant when the study ended.
Stigma receptivity and pollen longevity were determined by
bagging inflorescences still in bud from different plants (four for
stigma receptivity and 16 for pollen longevity), and performing
controlled pollinations when the inflorescences reached peak bloom.
The stigmas of four flowers (on the same inflorescence) that had
been open for only 1 day were treated with pollen from four flowers
on another plant that had been open 1, 2, 3 and 4 days,
respectively. Toothpicks were used to transfer the pollen. Stigmas
from 2, 3 and 4 day-old flowers were treated in a similar manner.
Treatments were replicated 4 times using a different male donor for
each replicate. Two flowers on each inflorescence were left
untreated as controls for detecting self-fertilization. Jeweler's
tags were used to label the treatment regime of each flower.
Inflorescences were rebagged immediately after treatment. Fruit set
was used as an indicator of stigma receptivity and pollen longevity.
Counts of fruit set per 4 replicates were organized in a 4  4
contingency table using age of stigma as columns and age of pollen
as rows. Fisher's exact test was used to analyze the table ( Ghent
1972). 4
contingency table using age of stigma as columns and age of pollen
as rows. Fisher's exact test was used to analyze the table ( Ghent
1972). |
|
Evaluation of the breeding system
Traditional breeding system studies usually involve emasculating
the flowers ( Schoen
& Lloyd 1992), but in the case of C. caseana this is
impractical. Because the anthers are appressed to the stigma, and
the anthers dehisce prior to, or simultaneously with, the opening of
the outer petals, anthers cannot be removed from an open flower
without moving pollen onto the stigma. Removing the anthers from a
bud would require cutting open both the outer and the inner petals;
the validity of pollination treatments after such damage would be
dubious. For that reason my outcrossing treatments did not include
emasculation. It is possible, in fact likely, that some self-pollen
would become attached to the stigma during outcrossing treatments.
Therefore I labeled this treatment outcross + self-pollinated.
Fourteen plants, each with a minimum of six flowering stems, were
selected for analysis of the breeding system. On each plant four
inflorescences were identified for experimental treatments; each
inflorescence was then randomly assigned to the treatment of: (i)
self-pollinated, (ii) outcross + self-pollinated, (iii)
open-pollinated, and (iv) non-pollinated. All 4 treatments were
performed on all 14 plants. Inflorescences (N = 56) were
bagged with green nylon netting while the flowers were in bud stage
to exclude pollinators. On the day of the treatment the bags were
removed and the pedicels of four open flowers (standardized by
flower age and position) on each experimental inflorescence were
marked with black ink pens (Sanford, Sharpie; Bellwood, Illinois,
USA). Pilot studies showed that marking a pedicel in this manner did
not affect normal development. Each of the four flowers was treated
in an identical manner.
For the self-pollination treatment the inner petals were
depressed (as shown in Fig.
1), a toothpick was used to press the anthers against both sides
of the stigma, and the netting was replaced. For the outcross +
self-pollination treatment fresh pollen, collected from more than 10
plants located 100-200 m from the experimental plant, was mixed and
applied to both sides of the stigma, again using a toothpick. The
netting was replaced. For the open-pollination treatment the netting
was simply removed, and for the non-pollinated treatment the netting
was removed for approximately 30 s and then replaced.
After 16 days the fruits were collected and dissected to
determine seed set. The seeds in each fruit were counted under a
dissecting microscope, and transferred to a coin envelope. Seeds
inside the envelope were oven dried at 40°C for 72 h and weighed
using an electronic analytical balance (Denver Instrument Company,
Arvada, Colorado, USA) to determine mean seed weight per fruit. The
seed weight per fruit for each of the four treated flowers on an
inflorescence was then pooled to determine the mean seed weight per
inflorescence.
Results were analyzed by two-way (treatment and plant) ANOVA ( Underwood
1997; p. 387). A separate analysis was carried out for each of
the dependent variables: (i) percentage fruit set per inflorescence,
(ii) mean number of seeds per fruit per inflorescence, and (iii)
mean seed weight per inflorescence. Differences between treatment
means for each variable were compared by the Tukey test ( Zar
1984). |
|
Pollinator observations
Pollinator species assemblages may vary due to time of day, time
of season, or the location (e.g. Herrera
1988). In order to get a broad idea of the visitors to C.
caseana flowers, I made observations throughout the flowering
season, at different times of the day (between 09.00 and 16.00
hours), and in two different locations during 1996. Observations
were made at Washington Gulch on 24, 25, 26 and 30 June ; 1, 2, 5,
7, 9, 10 and 12 July and 7 and 9 August. Observations were made at
Kebler Pass on 11, 16, 19 and 23 July and 4 August. Observations
were made by visually scanning each patch of plants in a study area.
If a pollinator was noted it was identified, by close visual
examination in the field, to species (and caste in the case of
bumblebees) and mode of foraging behavior - legitimate, primary
nectar robber or secondary nectar robber (sensu Inouye
1983). |
|
Pollinator effectiveness
To determine if flower visitors were indeed pollinators, 50
inflorescences still in bud were covered with bags made from green
mesh netting that excluded visitors. When the inflorescences were in
bloom, the bags were removed and the pedicels of all open flowers on
the inflorescence were marked (as in the breeding experiments).
After a visitor foraged on an experimental inflorescence, the
identity of the visitor and the number of flowers visited on the
inflorescence was recorded on a jeweler's tag. This method was
preferable to tagging the individual flowers visited because
hummingbird visits were so swift that it was difficult to see
exactly which flowers had been visited. After the 4 h observation
period the inflorescences were rebagged to prevent subsequent
visits. Unvisited inflorescences were considered to be controls and
were also rebagged. Sixteen days later the inflorescences were
collected and brought to the laboratory where fruit set and seed set
were determined. Seed set data are for number of seeds per fruit
formed. |
|
Nectar concentration, volume, and
accumulation
All nectar measurements were taken by inserting a 10  L
micropipette tube (Microcaps, Drummond Broomall, Pennsylvania, USA)
into the spur of the flower. For ease of handling, flowers were
first removed from the plant. Care was taken not to pierce the
corolla or otherwise contaminate the nectar with cell sap. Nectar
was drawn into the tube by capillary action. Volume was determined
by measuring the length of the filled tube with a digital micrometer
(Mitutoyo, Utsunomiya-shi, Japan) and converting the length
measurement to microliters. To measure nectar concentration, the
contents of the micropipette tube were emptied onto a portable
Bellingham & Stanley refractometer (Tunbridge Wells, UK)
modified to handle small volumes. Reported concentrations are from
the 106 flowers sampled on 2 and 3 July 1996, during sunny weather,
that had over 2 L
micropipette tube (Microcaps, Drummond Broomall, Pennsylvania, USA)
into the spur of the flower. For ease of handling, flowers were
first removed from the plant. Care was taken not to pierce the
corolla or otherwise contaminate the nectar with cell sap. Nectar
was drawn into the tube by capillary action. Volume was determined
by measuring the length of the filled tube with a digital micrometer
(Mitutoyo, Utsunomiya-shi, Japan) and converting the length
measurement to microliters. To measure nectar concentration, the
contents of the micropipette tube were emptied onto a portable
Bellingham & Stanley refractometer (Tunbridge Wells, UK)
modified to handle small volumes. Reported concentrations are from
the 106 flowers sampled on 2 and 3 July 1996, during sunny weather,
that had over 2  L of nectar. L of nectar.
The standing crop of nectar was measured by running a 35 m
transect parallel to the length of a C. caseana patch. At
each 5 m interval, one flower was collected from each of eight
different plants. Sampled flowers were all approximately 3 days old
and located on a terminal inflorescence. To determine mean nectar
accumulation per flower in the absence of visitors, I enclosed two
patches of C. caseana plants in screen tents (3.9 m  2.7 m 2.7 m
 2
m). In each patch 10 inflorescences were tagged. At 0 h (immediately
after enclosure), 24 h, 48 h, 72 h and 120 h, four flowers from each
inflorescence were removed and nectar volume was measured (N
= 80). I determined nectar accumulation by averaging the volume in
the four flowers sampled per inflorescence, and using those means
for each of the 20 inflorescences to calculate the grand means and
variance at each time period. 2
m). In each patch 10 inflorescences were tagged. At 0 h (immediately
after enclosure), 24 h, 48 h, 72 h and 120 h, four flowers from each
inflorescence were removed and nectar volume was measured (N
= 80). I determined nectar accumulation by averaging the volume in
the four flowers sampled per inflorescence, and using those means
for each of the 20 inflorescences to calculate the grand means and
variance at each time period. |
|
|
|
Flower phenology
Flowers begin blooming on the terminal raceme first, from the
bottom to the top (acropetally). On large, terminal racemes, the
bottommost flowers may be producing fruits while the uppermost
flowers are still in bud. In 1996-1998, in the locations of this
study, flowering began between 14 and 20 June. When an individual
flower opened it remained in good condition for approximately 4 days
(3.6 ± 1.3 days, mean ± SD; N = 101). The flower then
developed brown spots that grew larger each day; this phase lasted
for another 4 days (3.8 ± 1.2) until the corolla completely wilted
or fell from the inflorescence revealing a developing fruit or a
vacant pedicel.
There is no apparent spatial or temporal separation of female and
male function in C. caseana. In the stigma receptivity and
pollen longevity experiment at least one flower (from the 4
replicates) produced fruit in each of the treatments ( Table
1). The stigmas are receptive from the day the flower opens (day
1) at least until the flower begins to brown (day 4). Likewise,
pollen age (between 1 and 4 days) did not affect fruit set, and
pollen age was independent of stigma age in determining fruit set.
The flowers used to measure pollen longevity were not emasculated;
therefore self-pollen the same age as the stigma would have been
available in each treatment in addition to the applied pollen.
Despite this confounding factor, I have shown that 1-day-old pollen
is viable on a 1-day-old stigma, and 4-day-old pollen is viable on a
4-day-old stigma, and this also applies to situations intermediate
to these. Therefore, it seems likely that the pollen remains viable
from the time of anthesis, at least until the flower begins to
brown. |
|
Evaluation of breeding system
The flowers that were bagged, but not hand-pollinated, did not
set fruit. This treatment was removed from subsequent analyses. The
remaining three treatments: self-pollinated, outcross +
self-pollinated and open-pollinated, showed significant differences
in fruit set ( ANOVA, F
2,26 = 12.95, P< 0.005). In the
open-pollination treatment an average of 91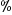 (N = 14) of the
treated flowers in an inflorescence set fruit ( Fig.
2). This was significantly different from the fruit set in the
self-pollinated and outcross + self-pollinated treatments (Tukey
test). In the flowers that were treated with self-pollen, 42 (N = 14) of the
treated flowers in an inflorescence set fruit ( Fig.
2). This was significantly different from the fruit set in the
self-pollinated and outcross + self-pollinated treatments (Tukey
test). In the flowers that were treated with self-pollen, 42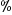 set
fruit, compared to 46 set
fruit, compared to 46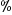 in the flowers receiving outcross +
self-pollen. These values were not significantly different from each
other. in the flowers receiving outcross +
self-pollen. These values were not significantly different from each
other.
In addition to differences in the number of fruits produced per
flower, there were also significant differences in the number of
seeds produced per fruit in the various treatments ( ANOVA, F 2,26 = 10.89,
P< 0.005). Open-pollinated and outcross + self-pollinated
flowers produced more seeds per fruit (5.0 ± 0.8 and 4.7 ± 0.8, mean
± 2 SE, respectively) than selfed flowers (2.9 ± 0.65; Fig.
3).
Treatment effects on mean seed weight were signifi-cant (F
2,26 = 3.75, P = 0.037). Mean seed weight from the
open pollinated inflorescences (0.985 ± 0.199 mg, N = 14) was
greater than from either the self-pollinated (0.796 ± 0.106 mg) or
the outcross + self-pollinated (0.850 ± 0.105 mg) inflorescences (
Fig.
4). Although the difference between the self-pollinated and
outcross + self-pollinated treatments was not significant, the
difference between the self-pollinated and the open-pollinated
flowers was (Tukey test). |
|
Pollinator observations
Two hundred and fourteen visitors were observed on the C.
caseana flowers, summarized in Table
2. The majority of the visitors (59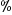 ) were Bombus
appositus, a long-tongued bumblebee. Bombus appositus
usually entered the flowers through the front to collect nectar
legitimately. In the process of nectar collection, the ventral
surface of the bee came into contact with the anthers of the flower.
Consequently, pollen would collect on the bee and the bee would
occasionally land, or hover, and groom the pollen into her pollen
baskets. Therefore, B. appositus foraging in this manner,
collected nectar and pollen simultaneously. In many hundreds of
observations from this and other studies on C. caseana, I
have never seen B. appositus foraging solely for pollen. I
captured four bees, removed their pollen loads, and released the
bees. In each case more than 99 ) were Bombus
appositus, a long-tongued bumblebee. Bombus appositus
usually entered the flowers through the front to collect nectar
legitimately. In the process of nectar collection, the ventral
surface of the bee came into contact with the anthers of the flower.
Consequently, pollen would collect on the bee and the bee would
occasionally land, or hover, and groom the pollen into her pollen
baskets. Therefore, B. appositus foraging in this manner,
collected nectar and pollen simultaneously. In many hundreds of
observations from this and other studies on C. caseana, I
have never seen B. appositus foraging solely for pollen. I
captured four bees, removed their pollen loads, and released the
bees. In each case more than 99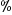 of the pollen they were carrying was
from C. caseana. In 8 of the pollen they were carrying was
from C. caseana. In 8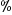 of the pollinator observations,
however, smaller B. appositus individuals - presumably
workers - collected nectar through existing holes in the nectar
spur. Using a pre-made hole in such a manner is called secondary
nectar robbing ( Inouye
1983). Bees foraging in this way did not contact the anthers,
therefore they did not collect pollen. of the pollinator observations,
however, smaller B. appositus individuals - presumably
workers - collected nectar through existing holes in the nectar
spur. Using a pre-made hole in such a manner is called secondary
nectar robbing ( Inouye
1983). Bees foraging in this way did not contact the anthers,
therefore they did not collect pollen.
The second most common visitor (29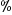 ) was Bombus
occidentalis, a short-tongued bumblebee. Most of these bees
behaved as primary nectar robbers - biting a hole in the back of the
corolla and inserting their proboscis through the hole to collect
nectar. Once holes were made by B. occidentalis, either B.
appositus (a long-tongued bee), B. occidentalis (a
short-tongued bee), or B. flavifrons (a medium-tongued bee),
could use the holes to collect nectar. The effects of corolla
perforation ( ) was Bombus
occidentalis, a short-tongued bumblebee. Most of these bees
behaved as primary nectar robbers - biting a hole in the back of the
corolla and inserting their proboscis through the hole to collect
nectar. Once holes were made by B. occidentalis, either B.
appositus (a long-tongued bee), B. occidentalis (a
short-tongued bee), or B. flavifrons (a medium-tongued bee),
could use the holes to collect nectar. The effects of corolla
perforation ( robbing robbing ) on forager behavior and
seed production are covered in detail elsewhere ( Maloof
2000). ) on forager behavior and
seed production are covered in detail elsewhere ( Maloof
2000).
Three percent of the visitors were hummingbirds (Selaphorus
rufus and Selaphorus platycerus) and on two occasions the
Gothic Swallowtail butterfly (Papilio zelicaon) was observed
collecting nectar from C. caseana.
From 1996 to 1998 I also made casual observations of visitors to
other C. caseana populations. At one population in Elkton,
approximately 2 km north of the Washington Gulch study site,
hummingbirds were more prevalent than they were in the regular study
sites. At a population in Yule Basin (39.00°N, 107.06°W; 3346 m)
Bombus nevadensis and B. kirbyellus, other
long-tongued bumblebees, were observed foraging legitimately, and
probably pollinating, alongside B. appositus. In the same
area, but at a higher elevation (3474 m) a hawkmoth (Hyles
lineata) was observed collecting nectar from C. caseana.
Another subspecies, Corydalis caseana ssp. cusikii,
was observed growing by Mores Creek Summit in the Boise National
Forest, Idaho. Of the 15 visitors observed there on 24 June 1997, 10
were B. appositus (legitimate pollinators), four were B.
occidentalis (nectar robbers), and one was a hummingbird. I
found it interesting that this suite of visitors was similar to the
suite observed in the study sites of C. caseana ssp.
brandegei, approximately 1000 km away. |
|
Pollinator effectiveness
Queens of B. appositus visited 5 of the 50 experimental
inflorescences. Between 2 and 5 flowers were visited on each
inflorescence (mean 3.6). None of the unvisited control flowers set
fruit, but 100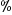 of the visited flowers set fruit.
Mean seed set was 3.9 seeds per fruit (N = 16, R =
1-7). Migratory Rufous Hummingbirds were also observed visiting five
inflorescences. They visited between three and 15 flowers on each
inflorescence (mean 9.6). None of the flowers visited by the
hummingbirds set fruit. of the visited flowers set fruit.
Mean seed set was 3.9 seeds per fruit (N = 16, R =
1-7). Migratory Rufous Hummingbirds were also observed visiting five
inflorescences. They visited between three and 15 flowers on each
inflorescence (mean 9.6). None of the flowers visited by the
hummingbirds set fruit. |
|
Nectar volume, accumulation and
concentration
Standing crop nectar volume, measured 9 July 1996, at 09.30
hours, ranged from 0.0 to 3.12  L. Mean nectar volume for the standing
crop was 0.60 ± 0.73 L. Mean nectar volume for the standing
crop was 0.60 ± 0.73  L (mean ± SD; N = 64). Figure
5 shows the temporal change in mean nectar volume over a 120 h
period. L (mean ± SD; N = 64). Figure
5 shows the temporal change in mean nectar volume over a 120 h
period.
Flowers had a mean sugar concentration of 35 ± 7.5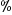 (mean
± SD; N = 106; r = 19-50 (mean
± SD; N = 106; r = 19-50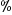 ). ). |
|
This is the first study conducted of flower longevity in C.
caseana. The flowers remain open, in good condition, for
approximately 4 days; they then develop brown spots and drop, or
wilt, after another 4 days. Similarly, C. cava flowers have a
life-span of approximately 9 days ( Olesen
1996). The flowers of C. ambigua last from 2 to 25 days
depending, in part, on the air temperature and whether or not the
flowers have been pollinated ( Yasaka
et al. 1998).
The results of this study indicate that the pollen is viable, and
the stigmas are receptive, for at least the first 4 days that the
flowers are open. There have been no other studies on pollen
viability or stigma receptivity in Corydalis.
The flowers that were bagged, but not hand-pollinated, did not
set fruit. This is consistent with the findings of Lloyd
& Schoen (1992) that autonomous modes of self-pollination
are rare in families with bilaterally symmetrical flowers such as
the Fumariaceae (the family to which C. caseana belongs).
Likewise, in the pollinator effectiveness observations, flowers that
received no visits did not produce fruits or seeds.
The results of the breeding study indicate that C. caseana
has a mixed breeding system; it is capable of pollinator mediated
self-fertilization as well as outcrossing. In self-fertile species
we often see spatial or temporal separation of male and female
function, presumably as a means to prevent autogamy (pollen transfer
within a single flower) and promote outcrossing. But in the case of
C. caseana, a self-fertile species, anthers dehisce pollen
onto a receptive stigma. How can the evolution of this mating system
be explained? It appears that C. caseana is selecting for
outcrossing by requiring a visit from a pollinator before
fertilization can occur. It is unclear exactly why a pollinator
visit is required for fertilization perhaps there is a stigmatic cuticle
that must be ruptured, as in the case of Medicago spp. ( Kreitner
& Sorensen 1985), before the pollen tubes can enter the
stigma. Whatever the exact mechanism is, it must promote
outcrossing, for wherever there are pollinators there is likely to
be at least some non-self pollen, and if this outcross pollen has
some advantage in rate of germination or tube growth, then
fertilization by outcross pollen could be expected to occur, even in
the case of a stigma with abundant amounts of self-pollen (see Spira
et al. 1992). Additional research is needed on these
questions. perhaps there is a stigmatic cuticle
that must be ruptured, as in the case of Medicago spp. ( Kreitner
& Sorensen 1985), before the pollen tubes can enter the
stigma. Whatever the exact mechanism is, it must promote
outcrossing, for wherever there are pollinators there is likely to
be at least some non-self pollen, and if this outcross pollen has
some advantage in rate of germination or tube growth, then
fertilization by outcross pollen could be expected to occur, even in
the case of a stigma with abundant amounts of self-pollen (see Spira
et al. 1992). Additional research is needed on these
questions.
The populations studied during this research do not appear to be
pollen limited. Fruit set in open pollination treatments was higher
(91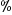 ) than fruit set in the
hand-pollinated trials (46 ) than fruit set in the
hand-pollinated trials (46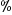 ), a typical measure of pollen
limitation. Why did the open-pollinated (control) flowers produce
more fruits than the selfed or outcrossed flowers pollinated by
hand? Young
& Young (1992) found similar results in 17 ), a typical measure of pollen
limitation. Why did the open-pollinated (control) flowers produce
more fruits than the selfed or outcrossed flowers pollinated by
hand? Young
& Young (1992) found similar results in 17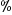 of the
cases they studied. Among the possible causes listed in their study
are: (i) at high densities (such as those created by
hand-pollination) pollen grains or pollen tubes may interfere with
each other, (ii) peak stigma receptivity may be missed by the
experimenter, or (iii) the bagging process itself may reduce seed
set. The open-pollinated flowers were left unbagged after treatment
so perhaps multiple visits, by pollinators, over a longer time
period were more effective at targeting peak stigma receptivity than
this experimenter's one-time pollen application. I don't have any
reason to believe that the bags interfere with fruit set, seed
number, or seed weight, but it is possible. The self- and
outcross-pollinated flowers were treated identically in every
respect except for the pollen they received, so comparisons between
those two treatments are uncomplicated by possible bagging effects. of the
cases they studied. Among the possible causes listed in their study
are: (i) at high densities (such as those created by
hand-pollination) pollen grains or pollen tubes may interfere with
each other, (ii) peak stigma receptivity may be missed by the
experimenter, or (iii) the bagging process itself may reduce seed
set. The open-pollinated flowers were left unbagged after treatment
so perhaps multiple visits, by pollinators, over a longer time
period were more effective at targeting peak stigma receptivity than
this experimenter's one-time pollen application. I don't have any
reason to believe that the bags interfere with fruit set, seed
number, or seed weight, but it is possible. The self- and
outcross-pollinated flowers were treated identically in every
respect except for the pollen they received, so comparisons between
those two treatments are uncomplicated by possible bagging effects.
Although the flowers are self-fertile the self-pollinated flowers
exhibited the lowest values for every parameter measured (fruit set,
seed number, seed weight), suggesting lower fitness due to
inbreeding depression. However, of the three parameters measured,
only the difference in seed number was statistically
significant.
In 1996 B. appositus was the most numerous visitor and the
only known pollinator in the study sites. Bombus occidentalis,
the nectar robber, was the second most numerous visitor ( Table
2). Drawing on what is known of other plant-pollinator
relationships, I would expect the exact numbers and perhaps even the
composition of the pollinator community to change through space and
time (e.g. Heinrich
1976; Herrera
1988; Traveset
et al. 1998). I have used 1996 as a Bombus occidentalis,
the nectar robber, was the second most numerous visitor ( Table
2). Drawing on what is known of other plant-pollinator
relationships, I would expect the exact numbers and perhaps even the
composition of the pollinator community to change through space and
time (e.g. Heinrich
1976; Herrera
1988; Traveset
et al. 1998). I have used 1996 as a  snapshot snapshot from
which I have made the following assumptions: (i) long-tongued
bumblebees are important pollinators of C. caseana, and (ii)
C. caseana sometimes shows evidence of high rates of robbing.
Additional experiments carried out at these study sites in 1997 and
1998 ( Maloof
2000) lend support to these assumptions. In each of those years
B. appositus was, again, the dominant visitor and at least
40 from
which I have made the following assumptions: (i) long-tongued
bumblebees are important pollinators of C. caseana, and (ii)
C. caseana sometimes shows evidence of high rates of robbing.
Additional experiments carried out at these study sites in 1997 and
1998 ( Maloof
2000) lend support to these assumptions. In each of those years
B. appositus was, again, the dominant visitor and at least
40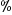 of the flowers were robbed.
of the flowers were robbed.
Because none of the 48 flowers visited by Rufous Hummingbirds set
fruit, I do not consider them to be pollinators of C.
caseana. More extensive studies should be done, however, to be
certain. It is possible that only a very small percentage of their
visits are effective, and there were no effective visits in my
sample. During the pollinator observation study resident
Broad-tailed Hummingbirds were observed visiting the flowers.
However, in the pollinator effectiveness study no Broad-tailed
Hummingbirds visited the experimental inflorescences, consequently,
I cannot say for certain that Broad-tailed hummingbirds are not
pollinators. Broad-tailed Hummingbirds generally have beaks in the
same size range as those of the Rufous Hummingbirds; the average
beak length in the two species differs by only 1 mm ( Calder
& Calder 1992; Calder
1993) therefore I would expect similar results from both
species.
The nectar produced by C. caseana rewards the pollinators
that are essential for fertilization and subsequent seed set. This
nectar contains a mean of 35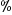 sugar, and in the absence of visitors
it accumulates at a rate of approximately 1 sugar, and in the absence of visitors
it accumulates at a rate of approximately 1  l per day. Flowers
protected from visitors for 120 h had a mean nectar volume of 6.22 ±
2.88 l per day. Flowers
protected from visitors for 120 h had a mean nectar volume of 6.22 ±
2.88  l (mean ± SD; N = 80). In
comparison, flowers recently exposed to visitors contained a mean
nectar volume of 0.23 ± 0.25 l (mean ± SD; N = 80). In
comparison, flowers recently exposed to visitors contained a mean
nectar volume of 0.23 ± 0.25  l, Fig.
5). Zimmerman
(1988) also measured the nectar standing crop in C.
caseana; on 5 August 1985, at 04.00 hours, and it was 0.27 ±
0.45(N = 103). The difference between nectar volumes in
unvisited and visited flowers indicates that visitors are removing
most of the nectar that the flowers produce. l, Fig.
5). Zimmerman
(1988) also measured the nectar standing crop in C.
caseana; on 5 August 1985, at 04.00 hours, and it was 0.27 ±
0.45(N = 103). The difference between nectar volumes in
unvisited and visited flowers indicates that visitors are removing
most of the nectar that the flowers produce.
The results of this study indicate that C. caseana is
dependent upon long-tongued bumblebee pollinators for reproduction,
and the pollinators are abundant and effective. |
| |
|
|
|
This study was made possible by financial support from Earthwatch
and its research corps, a Sigma-Xi grant in aid of research, and a
Faculty Development grant from Salisbury State University.
The author thanks David Inouye's Rocky Mountain Wildflower
Earthwatch teams of 1996, 1997 and 1998 for their assistance with
collecting data in the field. Special thanks to Heather O'Connor for
illustrating the flower. |
|
|
| • |
Calder W. A. (1993) Rufous Hummingbird.
The Birds of America 53. Academy of Natural Sciences,
Philadelphia.
|
| • |
Calder W. A. & Calder L. L. (1992)
Broad-tailed Hummingbird. The Birds of America 16. The
Academy of Natural Sciences, Philadelphia.
|
| • |
Ghent A. (1972) A method for exact testing of
2x2, 2x3, 3x3 and other contingency tables, employing binomial
coefficients. The American Midland Naturalist 88: 15
27.
|
| • |
Goldenberg D. M. (1992) Ecology of
Corydalis aquae-gelidae, a rare riparian plant. MSc
Thesis. Oregon State University, Corvalis (Unpubl.).
|
| • |
Goldenberg D. M. & Zobel D. B. (1997)
Allocation, growth and estimated population structure of
Corydalis aquae-gelidae, a rare riparian plant. Northwest
Science 71: 196 204.
|
| • |
Heinrich B. (1976) Resource partitioning
among some eusocial insects: Bumblebees. Ecology 57:
874 889.
|
| • |
Herrera C. M. (1988) Variation in mutualisms:
The spatio-temporal mosaic of a pollinator assemblage. Biological
Journal of the Linnean Society 35: 95 125.
|
| • |
Inouye D. W. (1983) The ecology of nectar
robbing. In: The Biology of Nectarines (eds B. Bentley &
Elias T.) pp. 153 173. Columbia University Press, New
York.
|
| • |
Kreitner G. L. & Sorensen E. L. (1985)
Stigma development and the stigmatic cuticle of Medicago
scutellata. Canadian Journal of Botany 63: 813
818.
|
| • |
Lanza J., Smith G. C., Sack S., Cash A.
(1995) Variation in nectar and composition of Impatiens
capensis at the individual, plant, and population levels.
Oecologia 102: 113 119.
|
| • |
Lloyd D. G. & Schoen D. J. (1992) Self-
and cross-fertilization in plants. II. The selection of
self-fertilization. International Journal of Plant Science
153: 370 380.
 |
| • |
Maloof J. E. (2000) The ecological effects of
nectar robbers, with an emphasis on the reproductive biology of
Corydalis caseana. PhD Dissertation. University of
Maryland, College Park Bell and Howell Information and Learning, Ann
Arbor, Michigan, USA.
|
| • |
Olesen J. M. (1996) From naïveté to
experience: Bumblebee queens (Bombus terrestris) foraging on
Corydalis cava (Fumariaceae). Journal of the Kansas
Entomological Society 69(Suppl.): 274 286.
|
| • |
Ownbey G. B. (1947). Monograph of the North
American species of Corydalis. Annals of the Missouri
Botanical Garden 34: 187 259.
|
| • |
Schoen D. J. & Lloyd D. G. (1992) Self-
and cross-fertilization in plants. III. Methods for studying modes
and functional aspects of self-fertilization. International
Journal of Plant Science 153: 381 393.
 |
| • |
Spira T. P., Snow A. A., Whigham D. F., Leak
J. (1992) Flower visitation, pollen deposition, and pollen-tube
competition in Hibiscus moscheutos (Malvaceae). American
Journal of Botany 79: 428 433.
|
| • |
Stern K. R. (1998) Corydalis. In: Flora of
North America North of Mexico, Vol. 3 (ed. Flora of North
America Editorial Committee) pp. 348 351. Oxford University Press,
New York.
|
| • |
Traveset A., Willson M. F., Sabag C. (1998)
Effect of nectar-robbing birds on fruit set of Fuchsia
magellanica in Tierra Del Fuego: A disrupted mutualism.
Functional Ecology 12: 459 464.
 |
| • |
Underwood A. J. (1997) Experiments in
Ecology. Cambridge University Press, Cambridge, UK.
|
| • |
Yasaka M., Nishiwaki Y., Konno Y. (1998)
Plasticity of flower longevity in Corydalis ambigua.
Ecological Research 13: 211 216.
|
| • |
Young H. J. & Young T. P. (1992)
Alternative outcomes of natural and experimental high pollen loads.
Ecology 73: 639 647.
|
| • |
Zar J. H. (1984) Biostatistical
Analysis. 2nd Edn. Prentice Hall, Englewood Cliffs.
|
| • |
Zimmerman M. (1988) Pollination biology of
montane plants: Relationship between rate of nectar production and
standing crop. The American Midland Naturalist 120: 50
57.
|
| |
| | |
|