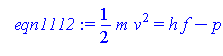
Variables
m = Mass of Electron
v = Velocity of Electron
h = Planck's constant
f = frequency
p = work function
l = wavelength
c = Speed of Light
Problem 11.20.b
In an X-ray photoelectron experiment, a photon of wavelength 121 pm eject an electron from the inner shell of an atom and it emerges with a speed of 56.9 Mm/s . Calculate the binding energy of the electron.
> eqn1112:=(1/2)*m*v^2=h*f-p;
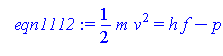
Variables
m = Mass of Electron
v = Velocity of Electron
h = Planck's constant
f = frequency
p = work function
l = wavelength
c = Speed of Light
Units
m = kg
v = m/s
h = J s
f = 1/s
p = J
l = m
> h:=6.62608*10^(-34);
![]()
> m:=9.10938*10^(-31);
![]()
In the problem we are given the speed of the ejected electron. Which is 56.9 Mm/s. Mm is a Megameter, which is a 10^6 meter. I want to first use a factor label method to get Mm/s in terms of m/s.
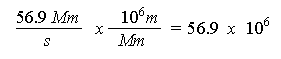 m/s
m/s
> 56.9*10^6;
![]()
5.69 x 107 is the velocity of the ejected electron, we can now assign this value to v (our variable for velocity, in m/s).
> v:=5.69*10^7;
![]()
We now have to get the wavelength in terms of frequency. But the given wavelength is given to us in picometers, so we will need to factor label the wavelength to get it in terms of meters.
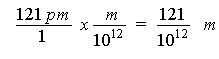
> 121/10^12;
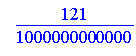
> evalf(121/1000000000000);
![]()
> l:=1.21*10^(-10);
![]()
We now have our wavelength in the correct units. To change wavelength into frequency we must manipulate the equation
c = λ f into c/λ = f.
Where c is the speed of light, λ is the wavelength, and f is frequency. Just plugging in the values c and v, we should get a usable frequency.
> c:=2.99792458*10^8;
![]()
> c/l;
![]()
Using the modified equation we found a frequency of 2.477623620 x 1018
hertz. We can now assign this to a variable.
> f:=2.477623620e18;
![]()
We now have all the variables except for what we are solving for p. We now are going to rearrange equation11.12 to solve for p.
.5 m v2 = h f - p
If we subtract (h f) from both sides leaving us with...
.5 m v2 - h f = -p
Now multiply both sides by -1 to be left with...
(.5 m v2 - h f)*(-1) = p
We can now just plug in all our values to get the end value for p, or the work function.
> ((.5*m*v^2)-h*f)*(-1);
![]()
With this we can conclude that the binding energy of the electron is 1.67062243 x 10-16 J .