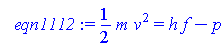Problem 3
For 11.20b, a plot (created in Maple) of velocity of electron vs possible incident wavelengths of light. The plot must be clearly labeled and all salient features must be clearly explained/dicussed -- be sure to explain what this plot "says" and why it "says" that.
We will be using equation 11.12 to make this graph. Because we are making a plot of kinetic energy vs frequency, we know that in terms of x,y, y will be the kinetic energy which is directly proportional to the velocity of the emitted electron, and x will be frequency, which is inversely proportional to the wavelength. We know this because in titles it is always Y vs X. To start we need to rearrange the equation and put everything in terms of y (kinetic energy).
> eqn1112:=(1/2)*m*v^2=h*f-p;
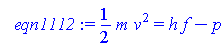
Another way to express this equation would be... KE= h f - p
Variables
m = Mass of Electron
v = Velocity of Electron
h = Planck's constant
f = frequency
p = work function
l = wavelength
c= Speed of Light
Units
m = kg
v = m/s
h = J s
f = 1/s
p = J
l = m
We need to set the variables as their constants.
> h:=6.62608*10^(-34);
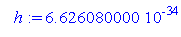
> m:=9.10938*10^(-31);
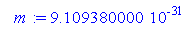
> c:=2.99792458*10^8;

The binding energy was found in the previous problem.
> p:=0.1670622430e-15;
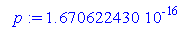
We know the values of h and p, so plugging in, we get.
> KE:=h*f-p;
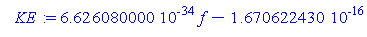
> KE;
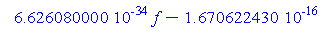
We have the kinetic energy of the electron in slope intercept form, so we can plot this.
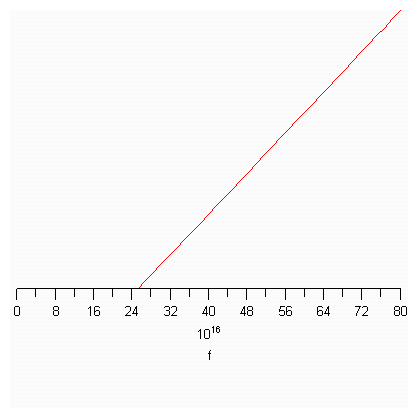
> plot(KE,f=0..1e17.8,y=0..1e-16.5);
We can find the x-intercept by letting KE equal 0, then solving for the frequency.
> KE:=0;

> f=(KE+p)/h;
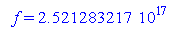
The x-intercept is 2.52 x 1017
Hz.
Slope intercept form is y = mx + b. Our y is the kinetic energy of the electron, our x is the frequency of the photon, m is Planck's constant, and b is the work function or the binding energy function. The x-intercept is the lowest frequency at which a photon can collide with the metal and possess enough energy to cause the metal to emit an electron. The electron will have a kinetic energy equal to the difference between the energy of the photon and work function.
Back to Main Page